

The geostan R package supports a complete spatial analysis workflow with Bayesian models for areal data, including a suite of functions for visualizing spatial data and model results. geostan models were built using Stan, a state-of-the-art platform for Bayesian modeling.
Introductions to the software can be found at r-spatial.org and in the package vignettes.
Features include:
For public health research, geostan complements the surveil R package for the study of time trends in disease incidence or mortality data.
There are two ways to install geostan: directly from the package github repository or from the Comprehensive R Archive Network (CRAN).
Using your R console, you can install geostan from CRAN:
install.packages("geostan")You can install geostan from github:
if (!require('devtools')) install.packages('devtools')
devtools::install_github("connordonegan/geostan")If you are using Windows and installing with
install_github, you may need to install Rtools first
(this is not needed when installing from CRAN). To install Rtools:
.exe file you just downloaded and
double-click to begin installation of Rtools.If you are using Mac and installing with install_github
then you may need to install Xcode Command Line Tools first.
All functions and methods are documented (with examples) on the website reference page. See the package vignettes for more on exploratory spatial analysis, spatial measurement error models, spatial regression with raster layers, and building custom spatial model in Stan.
To ask questions, report a bug, or discuss ideas for improvements or new features please visit the issues page, start a discussion, or submit a pull request.
Load the package and the georgia county mortality data
set:
library(geostan)
data(georgia)This has county population and mortality data by sex for ages 55-64, and for the period 2014-2018. As is common for public access data, some of the observations missing because the CDC has censored them to protect privacy.
The sp_diag function provides visual summaries of
spatial data, including a histogram, Moran scatter plot, and map. The
Moran scatter plot displays the values against a summary of their
neighboring values, so that the slope of the regression line gives a
measure of their degree of autocorrelation.
Here is a quick visual summary of crude female mortality rates (as deaths per 10,000):
# create adjacency matrix ("B" is for binary)
C <- shape2mat(georgia, style = "B")
#> Contiguity condition: queen
#> Number of neighbors per unit, summary:
#> Min. 1st Qu. Median Mean 3rd Qu. Max.
#> 1.000 4.000 5.000 5.409 6.000 10.000
#>
#> Spatial weights, summary:
#> Min. 1st Qu. Median Mean 3rd Qu. Max.
#> 1 1 1 1 1 1
# crude mortality rate per 10,000 at risk
mortality_rate <- georgia$rate.female * 10e3
# quick spatial diagnostics
sp_diag(mortality_rate, georgia, w = C, name = "Mortality")
#> 3 NA values found in x will be dropped from data x and from matrix w (nb: this disrupts row-standardization of w)
#> Warning: Removed 3 rows containing non-finite outside the scale range
#> (`stat_bin()`).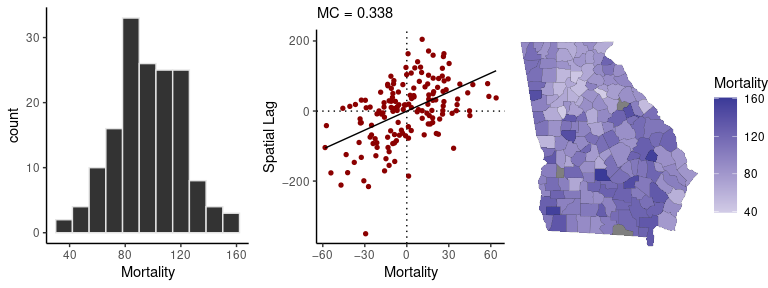
Mortality rates and other health statistics for counties are, in many cases, highly unstable estimates that cannot be relied upon for public advisories or inference (due to small population sizes). Hence, we need models to make inferences from small area data.
The following code fits a spatial conditional autoregressive (CAR)
model to female county mortality data. These models are used for
estimating disease risk in small areas like counties, and for analyzing
covariation of health outcomes with other area variables. The R syntax
for fitting the models is similar to using lm or
glm. We provide the population at risk (the denominator for
mortality rates) as an offset term, using the log-transform.
In our Georgia mortality data, three of the observations are missing
because they have been censored; per CDC criteria, this means that there
were 9 or fewer deaths in those counties. By using the
censor_point argument and setting it to
censor_point = 9, we can easily obtain estimates for the
censored counties (along with all the others) using models account for
the censoring process:
# prepare a list of data for CAR models in Stan
cars <- prep_car_data(C)
#> Range of permissible rho values: -1.661, 1
# fit the model to female mortality rates
fit <- stan_car(deaths.female ~ offset(log(pop.at.risk.female)),
censor_point = 9,
data = georgia,
car_parts = cars,
family = poisson(),
iter = 1e3, # no. MCMC samples
quiet = TRUE) # to silence printing
#> 3 NA values identified in the outcome variable
#> Found in rows: 55, 126, 157Passing a fitted model to the sp_diag function will
return a set of diagnostics for spatial models:
sp_diag(fit, georgia)
#> 3 NA values found in x will be dropped from data x and from matrix w (nb: this disrupts row-standardization of w)
#> Warning: Removed 3 rows containing missing values or values outside the
#> scale range (`geom_pointrange()`).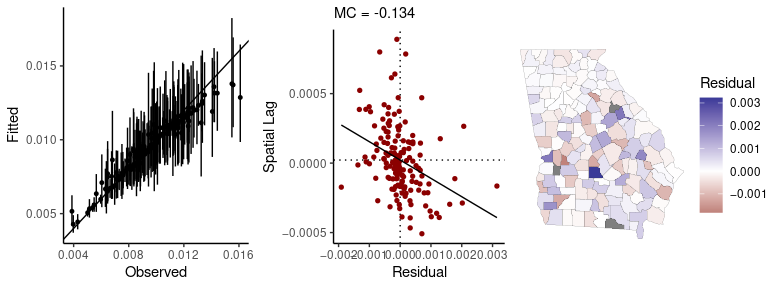
The print method returns a summary of the probability
distributions for model parameters, as well as Markov chain Monte Carlo
(MCMC) diagnostics from Stan (Monte Carlo standard errors of the mean
se_mean, effective sample size n_eff, and the
R-hat statistic Rhat):
print(fit)
#> Spatial Model Results
#> Formula: deaths.female ~ offset(log(pop.at.risk.female))
#> Likelihood: poisson
#> Link: log
#> Spatial method: CAR
#> Residual Moran Coefficient: -0.0031375
#> Observations: 156
#>
#> Inference for Stan model: foundation.
#> 4 chains, each with iter=1000; warmup=500; thin=1;
#> post-warmup draws per chain=500, total post-warmup draws=2000.
#>
#> mean se_mean sd 2.5% 20% 50% 80% 97.5% n_eff Rhat
#> intercept -4.677 0.003 0.092 -4.871 -4.734 -4.677 -4.619 -4.513 861 1.004
#> car_rho 0.924 0.001 0.058 0.784 0.883 0.936 0.973 0.996 1606 0.999
#> car_scale 0.456 0.001 0.036 0.391 0.424 0.454 0.486 0.532 1899 1.002
#>
#> Samples were drawn using NUTS(diag_e) at Wed Nov 13 16:09:50 2024.
#> For each parameter, n_eff is a crude measure of effective sample size,
#> and Rhat is the potential scale reduction factor on split chains (at
#> convergence, Rhat=1).To extract estimates of the county mortality rates from this, we
apply the fitted method - in this case, the fitted values
from the model are the estimates of the county mortality rates.
Multiplying them by 10,000 gives mortality rate per 10,000 at risk:
# mortality rates per 10,000 at risk
mortality_est <- fitted(fit) * 10e3
# display rates with county names
county_name <- georgia$NAME
head( cbind(county_name, mortality_est) )
#> county_name mean sd 2.5% 20% 50%
#> fitted[1] Crisp 101.89147 9.784748 83.87184 93.37621 101.37227
#> fitted[2] Candler 137.13841 15.643722 108.50033 123.57906 136.51359
#> fitted[3] Barrow 94.35971 6.364805 82.62612 88.73920 94.14574
#> fitted[4] DeKalb 59.74315 1.575741 56.77068 58.33325 59.71677
#> fitted[5] Columbia 53.34581 3.207432 47.33439 50.61504 53.26339
#> fitted[6] Cobb 54.12259 1.495041 51.24262 52.86109 54.12912
#> 80% 97.5%
#> fitted[1] 110.47617 122.04006
#> fitted[2] 150.26114 169.29720
#> fitted[3] 99.74677 107.51136
#> fitted[4] 61.13469 62.84502
#> fitted[5] 56.05293 59.83064
#> fitted[6] 55.38574 57.00559The mortality estimates are stored in the column named “mean”, and
the limits of the 95% credible interval are found in the columns “2.5%”
and “97.5%”. Here we create a map of estimates (with some help from
sf package):
library(sf)
# put estimates into bins for map colors
x <- mortality_est$mean
brks <- quantile(x, probs = c(0, 0.2, 0.4, 0.6, 0.8, 1))
est_cut <- cut(x, breaks = brks, include.lowest = TRUE)
# assign colors to values
rank <- as.numeric( est_cut )
pal_fun <- colorRampPalette( c("#5D74A5FF", "gray90", "#A8554EFF") )
pal <- pal_fun( max(rank) )
colors <- pal[ rank ]
# set plot margins
og=par(mar=rep(1, 4))
# get boundaries
geom <- sf::st_geometry(georgia)
# map estimates
plot(geom,
lwd = 0.2,
col = colors)
# legend
legend("right",
fill = pal,
title = 'Mortality per 10,000',
legend = levels(est_cut),
bty = 'n'
)
mtext('County mortality rates per 10,000, Georgia women ages 55-64', side = 3, font = 2)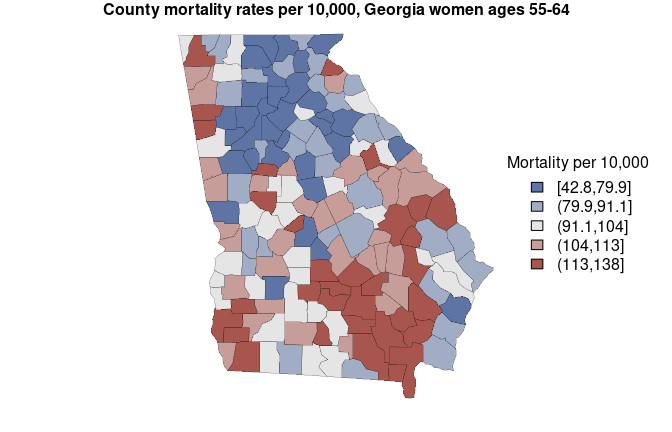
# reset margins
par(og)Using the credible intervals, we can complement our map with a point-interval plot:
# order counties by mortality rate
index <- order(mortality_est$mean, decreasing = TRUE)
dat <- mortality_est[index, ]
# gather estimate with credible interval (95%)
est <- dat$mean
lwr <- dat$`2.5%`
upr <- dat$`97.5%`
y <- seq_along(county_name)
x_lim <- c(min(lwr), max(upr)) |>
round()
og=par(mar = c(3, 0, 0, 0))
# points
plot(est,
y,
pch = 5,
col = 'gray50',
bty = 'L',
axes = FALSE,
xlim = x_lim,
ylab = NA,
xlab = NA)
# intervals
segments(x0 = lwr, x1 = upr,
y0 = y, y1 = y,
col = colors[ index ])
# x axis
axis(1, at = seq(x_lim[1], x_lim[2], by = 20))
mtext('County mortality rates per 10,000, Georgia women ages 55-64', side = 1, line = 2)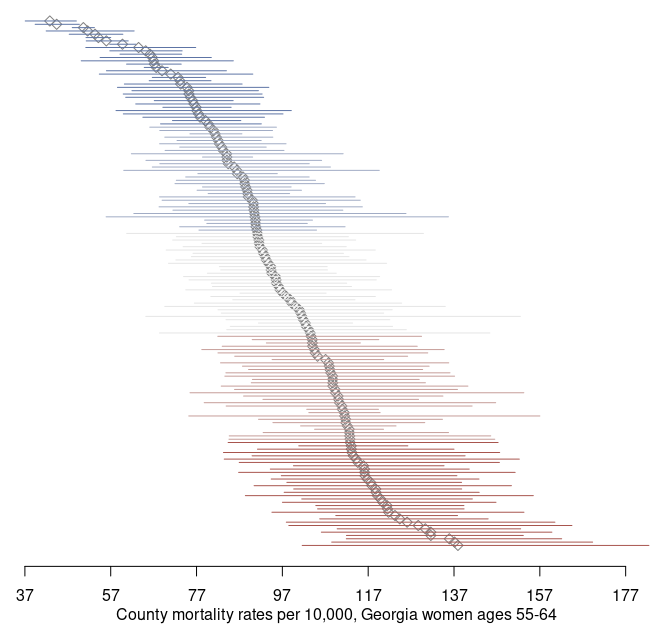
par(og)More details and demonstrations can be found in the package help pages and vignettes.
If you use geostan in published work, please include a citation.
Donegan, Connor (2022) “geostan: An R package for Bayesian spatial analysis” The Journal of Open Source Software. 7, no. 79: 4716. https://doi.org/10.21105/joss.04716.
@Article{,
title = {{geostan}: An {R} package for {B}ayesian spatial analysis},
author = {Connor Donegan},
journal = {The Journal of Open Source Software},
year = {2022},
volume = {7},
number = {79},
pages = {4716},
doi = {10.21105/joss.04716},
}